Abstract
Background: Myelodysplastic syndromes (MDS) are a heterogeneous group of disorders with variable outcomes. Acquired chromosome abnormalities in patients with myelodysplastic syndromes (MDS) are among the most valuable determinants of prognosis. However, chromosomal translocations are rare in MDS, and their prognostic implication is not well known. To elucidate factors driving chromosomal translocations in MDS, we compared the frequency of MDS patients with and without prior exposure to DNA damaging cytotoxic therapies.
Methods: We evaluated diagnostic bone marrow metaphase cytogenetic data of MDS patients enrolled in South Australian MDS/AML registry data. Appropriate Institutional Review Board approval was obtained. Diagnosis and subclassification of MDS were done according to World Health Organization (WHO) 2016 criteria and risk stratified according to the revised International Prognostic Scoring System (R-IPSS) (Greenberg et al., 2012). Standard G-banding method was used for cytogenetic analysis.
To decipher changes induced by cytotoxic therapy (CT) from that of myeloid neoplasm in general, we compared cytogenetic profile of three carefully selected cohorts: (i) tMN, in which a myeloid neoplasm occurred in a cancer survivor following exposure to cytotoxic (n = 206, 15.5%); (ii) myeloid neoplasm developing in unrelated cancer survivors with NO prior exposure to CT (a diagnosis of primary MN and another cancer; pMN+Ca) (n =148, 12%). This would include myelodysplastic syndrome or acute myeloid leukemia diagnosed in patients managed with surgical resection of breast cancer and NO exposure to CT; (iii) primary MDS without preceding independent cancer and/or exposure to CT (pMDS) (n = 893,72.5%)
Results: The median age of 1247 patients analyzed was 72.50 (IQR 64.9 to 79) and 64% patients were male. Majority of patients were diagnosed with pMDS (n=893; 72.5%), while 206 (15.5%) and 148 (12%) were classified as t-MN and pMN+Ca respectively.
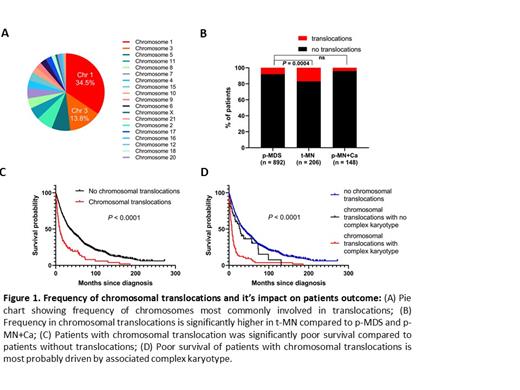
Ten percent (114/1247) patients harbored translocations at the time of diagnosis. The most frequent chromosomes involved in translocation are chromosome 1 (37/114, 34.5%), 3 (15/114, 13.8%), 5 (6/114= 5.3%) (Fig.1A). Most common chromosome partners for translocation with chromosome 1 were chromosome 3, 7 and 21.
The frequency of chromosomal translocations in patients with monsomal (55/114, 48%) and complex (70/114, 61%) karyotype were higher than patients without. There is a clear relationship in incidence of chromosomal translocations with increasing IPSS-R scores. The frequency of translocations in the Very high risk IPSS-R risk category was significantly higher compared to patients with Very low risk category (27% vs.1.1%).
Importantly, frequency of chromosomal translocations was significantly higher in t-MN as compared pMN+Ca and P-MN patients who were not exposed to DNA damaging cytotoxic therapies prior to their diagnosis (17% vs. 4% vs. 8%, P=0.0004; Fig 1B). Notably, the survival of patients with chromosomal translocation was significantly poorer as compared to patients without translocation (Fig.1C). Poor OS of patients with translocation is most probably due to association with complex karyotype (Fig.1D).
Conclusion: This comprehensive analysis demonstrates that chromosomal translocations are observed in 10% of MDS patients. Chromosomal translocations are significantly higher in patients who had prior exposure to cytotoxic therapy as compared to patients without.
Hiwase: Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal